In this first post we will be looking at the EU initiative exploring the introduction on an electronic Product Information (ePI) for human medicines authorized in the EU. We will also be looking at how the eLeaflet solution fro myHealthbox performs against the requirements set forth by this EU ePI initiative.
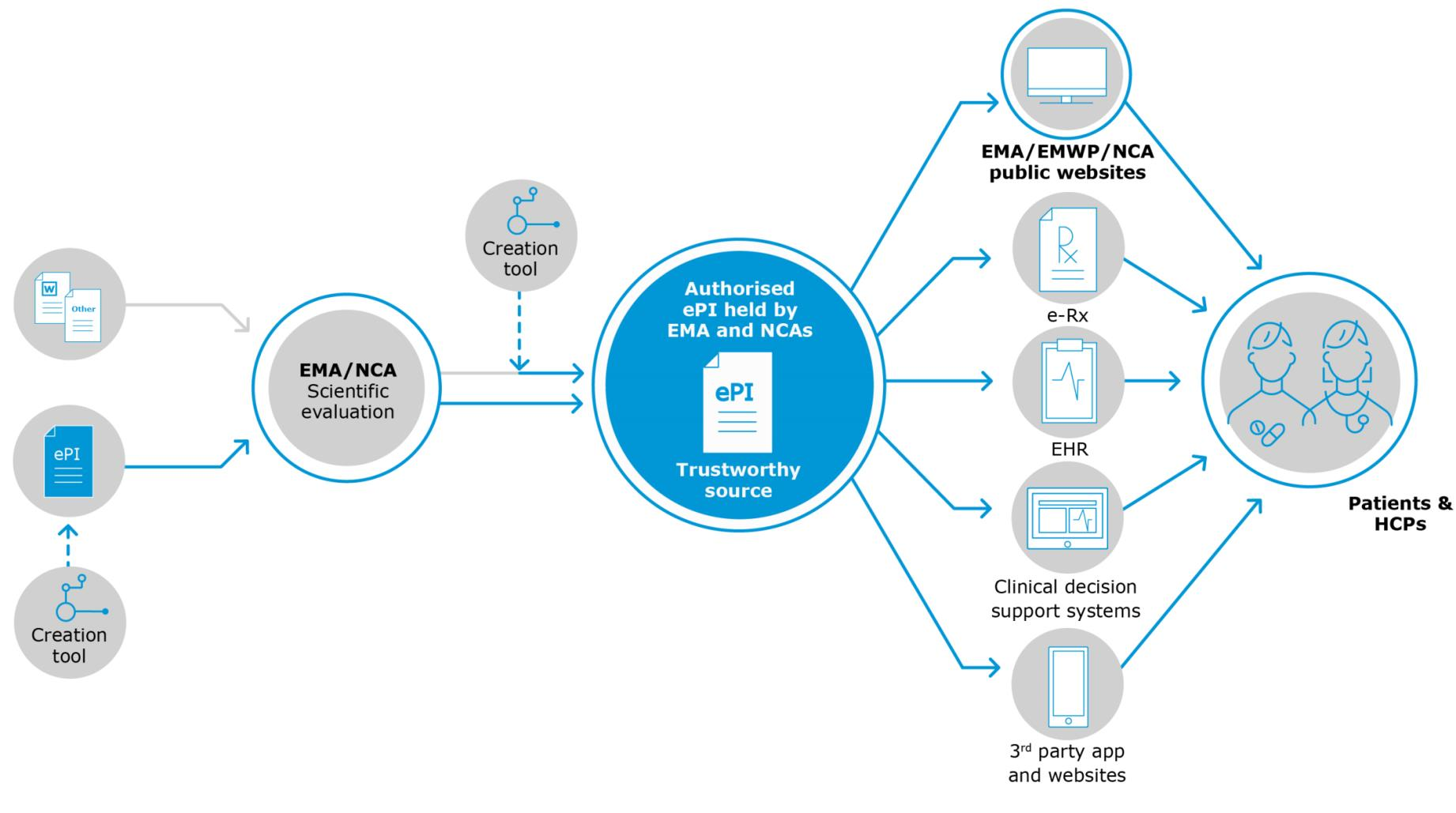
Specifically Part 1 of this series will look at the "status quo" for this initiative, the rationale for introducing this technology and the features expected from an ePI implementation which have been divided into 3 categories:
- those relating to ePI data (this article);
- those relating to ePI user experience (Part 2 available here);
- potential future functionalities that could be added on to ePI once it is established (Part 3 available here);.
The roadmap (so far)
March 2017
- The European Commission (EC) publishes its report on current shortcomings in the summary of product characteristics (SmPC) and the package leaflet (PL) and how they could be improved in order to better meet the needs of patients and HCPs.
November 2017
- The European Medicines Agency (EMA) publishes an action plan detailing the actions needed to meet the objectives set out in the EC report. One of the actions is to explore how ePI could facilitate access of EU citizens to the information in the PL and SmPC.
- EMA launches a survey seeking information from stakeholders in order to map ePI initiatives underway in the EU.
![]()
July 2018
- The Heads of Medicines Agencies (HMA) and representatives from national competent authorities (NCAs), the pharmaceutical industry, and patient and HCP organisations meet in Madrid to gather key stakeholders’ views on ePI.
September 2018
- EMA’s Patients’ and Consumers’ Working Party (PCWP) and Healthcare Professionals’ Working Party (HCPWP) are updated on ongoing work and draft key principles for ePI in the EU are discussed at their September meeting.
October 2018
- Virtual group discussions are held by representatives from the pharmaceutical industry’s Inter-Association Task Force (IATF) and the NCAs of France, Iceland, the Netherlands, Norway and Spain to draft features and use cases for a common standard for ePI.
November 2018
- The draft key principles for ePI are discussed at a meeting of the Coordination Group for Mutual Recognition and Decentralised Procedures - Human (CMDh).
- EMA-HMA-EC workshop on ePI takes place at EMA’s premises in London on 28 November. A diverse group of stakeholders, including patients, HCPs, academics, not-for-profit organisations and pharmaceutical-industry representatives attend the workshop.
January 2019
- A report, video and presentations from the workshop are published. The main output of the workshop, the document ‘Electronic Product Information for Human Medicines in the EU – Draft Key Principles’, is released for a 6 month public consultation.
January 2020
- A document describing the key principles to develop the ePI has been published by EMA, HMA and The European Commission. The guideline set the basis of the common technical standards to be used to overcome the issue of interoperability. The expected impact also includes a reduced time to work and make available regulatory variations. This document now represent EMA-HMA-EC guidance on ePI and forms the basis of follow-up implementation plans for ePI. The document is called: "Electronic product information for human medicines in the EU: key principles"
The features
ePI data
Open data that can be reused in other tools
ePI data should be freely available for use and reuse so that they become a resource for third parties, such as researchers, developers, organisations and businesses, as well as authorities outside the EU.
The eLeaflet solution from myHealthbox is based on open, web based, standards. Information is freely available and distributable.
Linked from medicine package
ePI should be accessible directly from the medicine package, for example by scanning a barcode on the package.
The eLeaflet solution from myHealthbox already supports a variety of codes available on packaging as well as text searches based on barcodes. The most common implementations support a QR code which in most cases does not require a dedicated application or reader but can be scanned in quickly from any smartphone.
Batch-specific ePI
Some changes to a medicine, such as a change to an excipient, could mean that a different ePI would be valid for different batches of a medicine. For example, older batches of a medicine that are still ‘on the shelf’ could have a different ePI to newer batches being released from the manufacturer. Therefore, it should be possible to link each medicine package in a particular batch to the correct, batch-specific ePI.
The eLeaflet solution from myHealthbox already supports this feature, an eLeaflet can be linked to a specific product or to a specific batch of products (batches must be identified by unique numbers). A search for the product will present the user with a list of batches where each batch may have different product information.
History of PI updates
ePI should support versioning, meaning that it should be possible to access historical versions of the ePI and note the changes that have taken place over time.
The eLeaflet solution from myHealthbox already supports this feature which is available on the myHealthbox search engine (only for users with a full license) or via APIs.
Data security
ePI content should be secure and protected against unauthorised changes.
The eLeaflet solution from myHealthbox implements a very strict protocol to guarantee that data is packaged in a way that cannot be modified.
The full list of features includes:
- support for a digital signature (checksum) to sign content
- a content verification procedure/API
- support for a blockchain notary service to implement content certification and verification.
Data privacy
Any use of ePI involving collection of personal data should comply with data protection legislation to ensure that patient privacy is upheld and that this is done legally.
The eLeaflet solution from myHealthbox does not ask nor use any personal information unless strictly necessary and only after explicit consent from the user. Features that require input of personal data like the "Pill Reminder" and the "Dosage Calculator" follow all existing privacy regulations in regards to data collection and usage. All analytics data is anonymized.
More information about the eLeaflet solution from myHealthbox is available on the eLeaflet website