ePi: an industry perspective
Industry Proposes Phased Rollout of ePI for Patient Safety and Environmental Sustainability
On 8 January 2025 the Inter-Association Taskforce (IATF) on electronic Product Information (ePI), a collaboration between the industry associations AESGP (Association of the European Self-Care Industry), EFPIA (European Federation of Pharmaceutical Industries and Associations) and Medicines for Europe published a series of position papers advocating for the implementation of electronic Product Information (ePI) and improvement of the patient leaflet content.

The papers show how, by transitioning to ePI, patients, healthcare professionals (HCPs), and civil society will benefit from the most up-to-date, accessible medicinal information, ensuring safer use of medicinal products.
Through these papers the industry is expressing their position on:
- how to move towards ePI and the progressive removal of paper package leaflets,
- how to practically implement ePI from a technological point of view,
- how the content of product information can be improved and
- how the use of multi country packs can be stimulated.
KEY HIGHLIGHTS
- ePI Phasing-In, Paper Phasing-Out: The gradual phasing in of ePI is proposed to be fully operational within 4 years after entry into force of the revised General Pharma Legislation and will precede the phasing out of paper leaflets. This will ensure patients have continuous access to critical medicinal information via secure, harmonised digital platforms. Existing ePI platforms such as National Competent Authority and Industry websites and compendia could be used as solutions to initiate the transition before ePI becomes fully available on the EMA/HMA portal. Phasing out paper in self-administered products will be more gradual than for HCP-administered products due to individual needs, administrative capabilities and product specific requirements.
- Improving PIL: Patient information leaflets would greatly benefit from layout and readability improvements. There are several proposals to benefit correct safe use of medicinal products, by delivering clear information to level up health literacy.
- Patient Safety and Digital Access: With 90% of EU citizens regularly accessing the internet Eurostat datasets : Statistics | Eurostat (europa.eu), ePI will allow for availability of up-to-date leaflets, interactive elements, personalised content, and more accessible formats such as large print or multimedia. However, alternatives for those without internet access will be retained to guarantee inclusivity.
- Safeguarding Availability in Small Markets: Multi-country packs, which are simplified by the use of ePI, language exemption and harmonised labelling requirements, will improve the availability of medicines across Europe particularly in smaller markets, reducing logistical burdens and fostering greater supply chain agility.
- Enhancing Regulatory Efficiency: The ePI platform is designed to streamline regulatory processes, reducing administrative burdens for both pharmaceutical companies and health authorities. The centralised EMA portal will serve as a single source of trustworthy information, fostering transparency and regulatory efficiency across the EU.
List of topics
The documents outline a strategic shift from current paper leaflets towards a more patient-centric content and accessible, environment-friendly digital alternative, designed to optimise pharmaceutical operations while keeping patient safety at the forefront.
The main topics discussed in the papers are:
- Phasing in of Electronic Product Information and Phasing Out of the Paper Package Leaflet
- Alternative Ways of Providing the Printed Package Leaflet of Medicinal Products
- Adding Disposal Information on the Labelling of Medicinal Products.
- Facilitating Medicines Availability and Environmental Benefits Through Language Exemptions and Electronic Product Information (ePI).
- Proposals to Support Multi-Country Packs and Simplify the Supply Chain
Phasing in of Electronic Product Information and Phasing Out of the Paper Package Leaflet
The implementation of electronic Product Information (ePI) and the removal of the paper package leaflet provides significant advantages for patients, healthcare professionals, industry, regulators and the environment by offering accessible and up-to-date information on medicines in an accessible digital format. ePI also strengthens supply chain agility and is a unique opportunity to mitigate and prevent shortages while significantly contributing to environmental sustainability.
This is the IATF proposal for implementation:
- IATF underscores that the phasing in of ePI (which is currently already taking place) should precede the phasing out of paper package leaflets. Existing ePI platforms such as National Competent Authority and Industry websites and compendia could be used as solutions to initiate the transition before ePI becomes fully available on the EMA/HMA portal.
- The phasing out of the paper package leaflet should start with products not intended for self- administration (Healthcare Professional (HCP)-administered products) first in all Member States and immediately after entry into force of the new legislation.
- While the landscape of internet access among EU citizens strengthens the case for the widespread adoption of ePI, it is crucial to continue providing accessible medicinal information to the small minority without regular internet access or with limited digital skills. Therefore, a solution that strikes a balance between completely removing paper and fully retaining paper, such as printing at the point of dispensation, seems to be one of the best solutions at the moment.
Are EU citizens ready for the use of ePI, and is the paper package leaflet still required?
The readiness of EU citizens for the adoption of electronic Product Information (ePI) is supported by encouraging Eurostat statistics, which reveal that internet access among EU citizens (at least once a week) stands at 90% in 2023 (72% in 2014) and continues to rise2. This trend is expected to significantly increase in the coming years, with projections indicating that by 2034, the percentage of EU citizens regularly accessing the internet will increase to 97% overall, and to 87% among those aged 65-74.
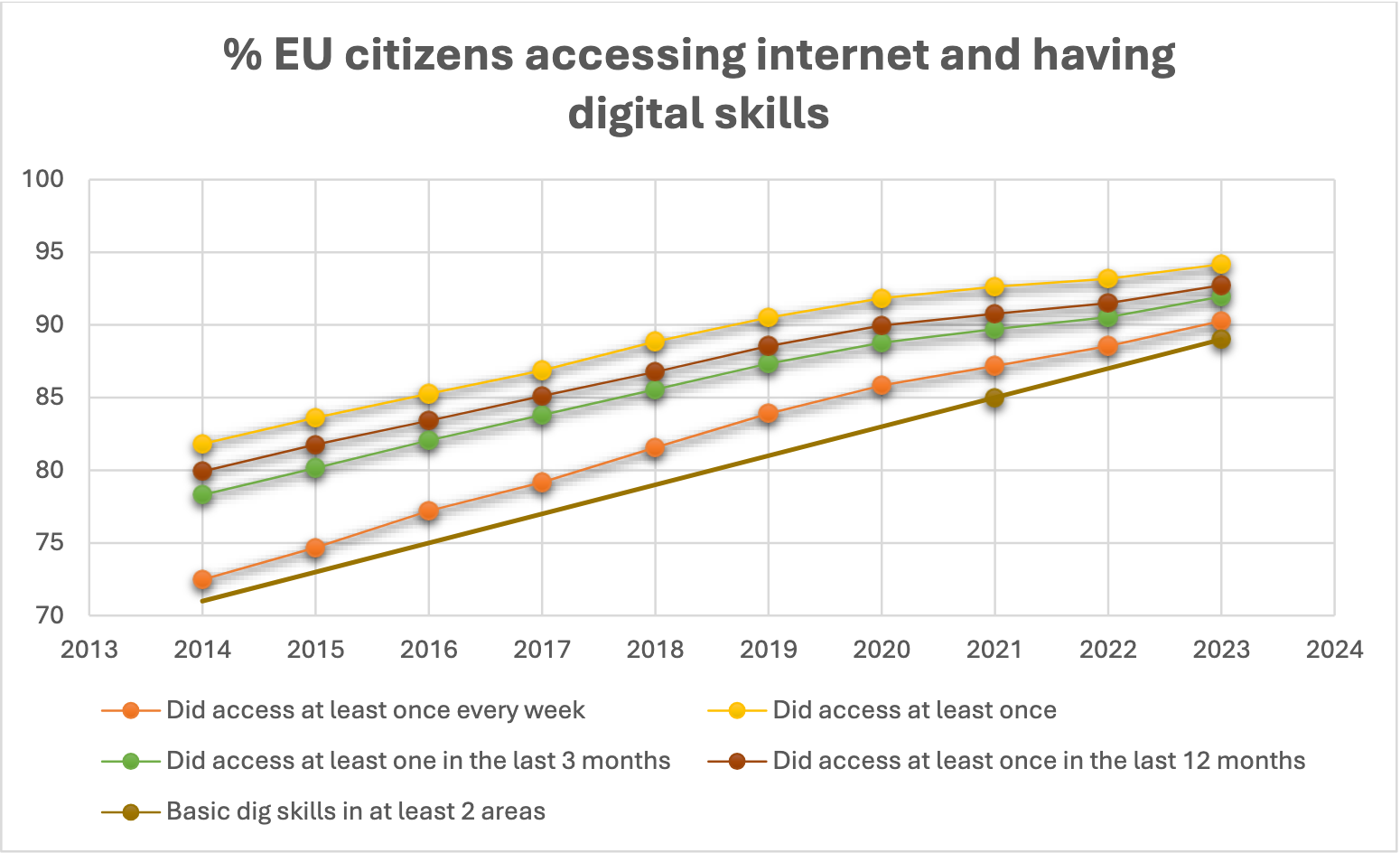
Despite the positive trajectory towards universal internet access and increased digital skills, the pharmaceutical industry remains committed to ensuring that no patient is left behind in the transition from paper to electronic product information.
In conclusion, while the landscape of internet access among EU citizens strengthens the case for the widespread adoption of ePI, it is crucial to continue providing accessible medicinal information to the small minority without regular internet access. However, maintaining the paper leaflet entirely for this ever-decreasing minority is disproportionate. Therefore, a solution that strikes a balance between completely removing paper and fully retaining paper, such as printing at the point of dispense seems to be one of the best solutions at the moment.
Alternative ways of providing the printed package leaflet of medicinal products
Three promising approaches are:
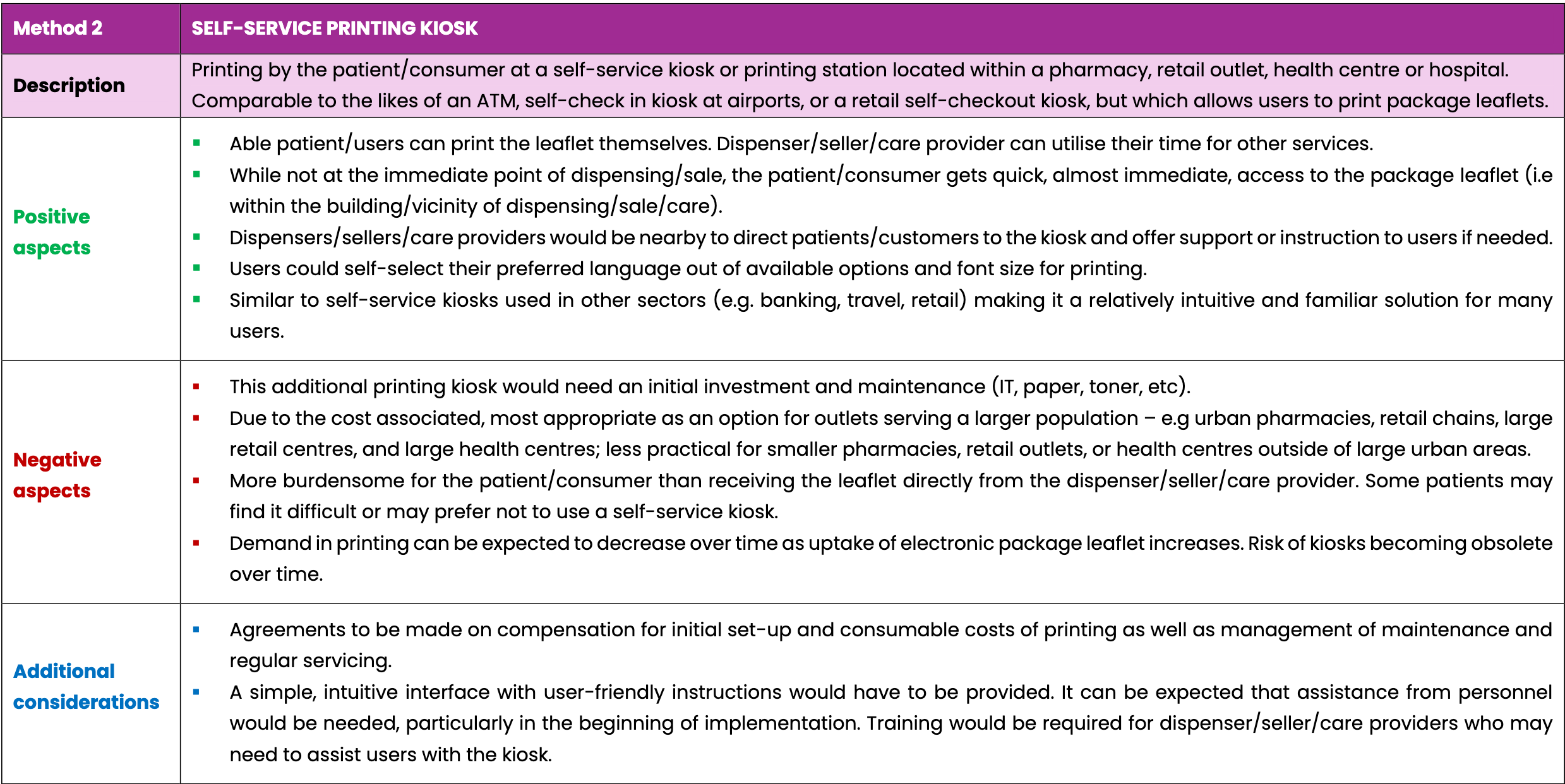
- Printing by a professional such as the medicinal product dispenser/seller or care provider, (e.g. at a pharmacy, health care centre, patient or elderly care facility)
- Printing by the patient at a self-service printing kiosk located within a pharmacy, retail outlet, health centre or hospital, and
- Printing by the patient at a self-service kiosk at a community printing hub in a suitable public location
All three options anticipate using scanning technologies with existing 2D data matrix codes or linear barcodes on the medicinal product packaging. This will safeguard that the correct leaflet for the dispensed or sold medicinal product is always printed, avoiding potential errors from manually searching for a specific leaflet.
Other possible solutions that were considered include courier delivery of package leaflets upon prescribing, and the delivery of printed resources by the manufacturer.
The tables below compare three possible ways to provide a printed copy of package leaflets to patients and consumers of medicinal products upon request. The three options listed are not exhaustive. This considers a future scenario where package leaflets are provided electronically only and are no longer required inside the packaging of each medicinal product.


Disclaimer
This post is a summary highlighting the main points put forward by various industry associations in relation to the introduction of the digital leaflet (eLeaflet) for healthcare and medicinal products. The content of this post does not necessarily reflect myHealthbox position in relation to these topics.
References
AESGP is the Association of the European Self-Care Industry, is a non-profit organisation that represents the manufacturers of non-prescription medicines, food supplements, and self-care medical devices in Europe, an area also referred to as “self-care” or “consumer healthcare” products.
EFPIA is the European Federation of Pharmaceutical Industries and Associations (EFPIA) represents the biopharmaceutical industry operating in Europe.
Medicines for Europe began over 20 years ago as the European Generic Medicines Association (EGA) with the goal of representing the emerging generic industry, and later growing to include bio-similar medicines to its portfolio. Medicines for Europe represents the pharmaceutical companies supplying the largest share of medicines across Europe and is the voice of the generic, bio-similar and value added industries.