Product information plays a pivotal role in ensuring patients’ understanding of their treatments while also supporting HCPs in their decision making. For product information to become more effective, new, digital-enabled tools are key components in enabling more effective use of available treatments and helping raise overall health literacy. These tools will facilitate a swifter access, better understanding and improved usability.
One emerging trend that fits in this category is electronic labelling, or e-labelling. E-labelling is defined as the dissemination of approved product information for medicinal products in a dynamic digital format allowing for the development of personalised product information based on the needs of the patients and healthcare professionals

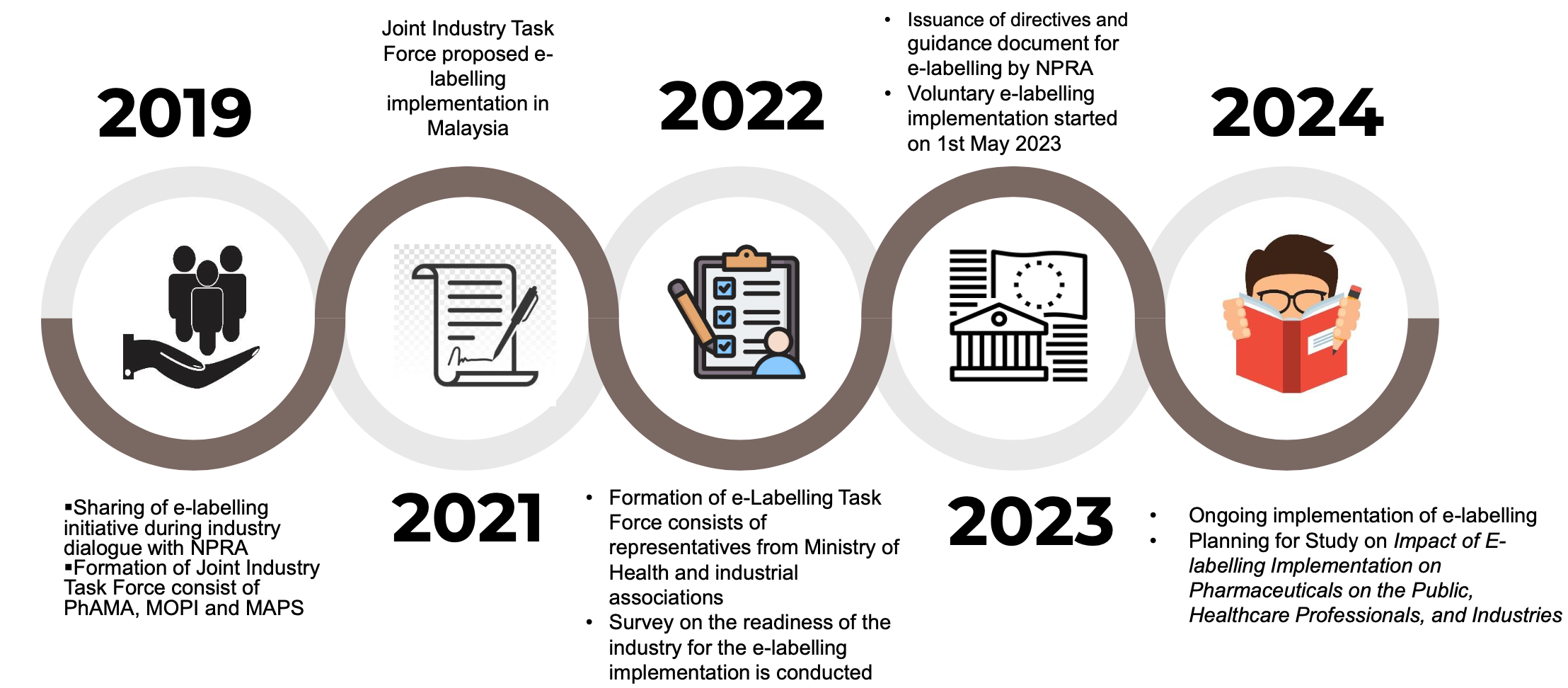
Approximately half of the countries in Asia have published product information in PDF format on their Health Authority (HA) websites. In Malaysia, e-labelling is proposed by industry since 2019 to be line with the healthcare digitalization initiatives which was discussed in International Pharmaceutical Regulators Programme (IPRP) and International Conference of Drug Regulatory Authorities (ICDRA) where e-labelling and reliance have been the main topic discussed. The implementation of e-labelling is in line with the global advancement in digital healthcare and e-labelling has become one of the emerging trends that is moving at different speeds around the world. Several countries have adopted e-labelling as means to provide patients and HCPs easy access to the latest product information.
E-Labelling Milestones in Malaysia
Survey on Industry Readiness: Main findings
READINESS ON E-LABELLING IMPLEMENTATION BY Q3 2022
- 20.5% ready
- 79.5% not ready due to:
- The current product label has been printed in a large amount
- Need more time to re-design label to include QR code
- Worry about variation approval time
- The readiness of the hosting site
WHEN DO YOU ANTICIPATE READINESS COULD BE ATTAINED?
- 82.3% ready to implement between 2023 - 2025
- 17.7% ready to implement by Q32022
WHAT IS YOUR PREFERRED E-LABELLING HOSTING SITE?
- 52.6% preferred NPRA
- 43.6% preferred company or 3rd party
- 3.8% has no preference
E-LABELLING REQUIREMENTS IN MALAYSIA
E-LABELLING DEFINITION
E-labelling is defined as the provision of an approved product information that includes the package insert (PI) and/or Consumer Medication Information Leaflet (RiMUP) electronically via a machine readable Quick Response (QR) code on the outer carton/inner label of the product that links to the NPRA QUEST system.
When PI and/or RiMUP is distributed via e-labelling, physical printed copies may also be distributed with the product. It is the responsibility of the Product Registration Holder (PRH) to provide the physical printed copies when it is required.
VOLUNTARY IMPLEMENTATION TIMEFRAME
Directive of implementation: 11 April 2023 Voluntary implementation period : 1 May 2023 – 31 December 2026
IMPLEMENTATION SCOPE
The implementation of e-labelling is voluntary and applies to new drug products, biological and generic products containing scheduled poisons for human use only.
IMPLEMENTATION METHOD
- For a new product: as part of product dossier.
- For an existing product: Minor Variation Notification
Guidelines document: GUIDELINE ON ELECTRONIC LABELLING (E-LABELLING) FOR PHARMACEUTICAL PRODUCTS IN MALAYSIA
DATA CARRIER: QR CODE
- The QR code may be displayed on the outer carton or inner label
- The QR code may be printed or affixed onto the outer carton/inner label using a stick-on label
DATA FORMAT: PDF
E-labelling shall be presented in a QR code on the outer carton/inner label of the product that translates to NPRA QUEST3+ page which displays the same product information in a pdf format.
The format would allow optimised viewing on any electronic devices such as smartphones/ laptops/ tablets.
Product information in video format is currently not allowed
HOSTING SITE: NPRA QUEST SYSTEM
The maximum capacity of product information (e-labelling) to be uploaded and hosted in QUEST3+ system shall not exceed 5MB
NEXT STEPS
Cross-Platform Compatibility
Designing e-labelling solutions that are compatible with a wide range of devices, operating systems, and software applications enhances interoperability.
Standardised Formats
Adopting standardised formats for e-labelling data is essential for interoperability. Common formats ensure that information can be easily exchanged and understood by different systems and devices
Medication Management
- E-labelling integration can enable healthcare providers, consumers and patients to access comprehensive medication information electronically
- This includes details such as dosage instructions, contraindications, and potential drug interactions
Sources
- GUIDELINE ON ELECTRONIC LABELLING (E-LABELLING) FOR PHARMACEUTICAL PRODUCTS IN MALAYSIA
- PhAMA: Pharmaceutical Association of Malaysia
- MOPI: The Malaysian Organisation of Pharmaceutical Industries
- MAPS: Malaysian Association of Pharmaceutical Suppliers