e-Labelling and eLeaflets in the APAC region
The Future of Drug Information in ASIA: an industry perspective
The availability of the latest product information on publicly accessible websites can be an important first step in improving patient safety and trust in medicines and can help accelerate the transformation process from paper labelling to e-labelling. Improved accessibility on public websites enhances user experience by improving navigation around the product information, can increase understanding of the correct usage of medicines and gives better familiarity with the safety and efficacy profile of the drug compared to more conventional printed labelling materials.
The Role of Product Information (the “Labelling”)
The product information (the “labelling”) is a key component of the submitted dossier but also a crucial communication tool. A variety of formats (paper, electronic) and types (patient, HCP) are distributed according to national requirements. It is also a critical risk minimisation measure communicating benefit/risk and usage instructions
Depending on the country, the pack may contain Healthcare Professional Information (e.g. U.S., Japan and India) or patient information (e.g. EU); in Asia, most commercial packs contain labelling targeted at HCPs.
Digital brings a shift in thinking
Much of the focus in regards to labelling has so far been around negotiation of the content and maintenance of the product information post-approval. Little attention has been paid to how the label is being accessed, used, understood and adhered to in real life. With digital the focus is now moving towards the “customer experience”.
What is e-Labelling
When we refer to e-Labelling we really refer to a list of mandatory or optional implementations:
- Availability of the latest product information on a publicly accessible website (i.e. product information is easily accessible online).
- Information available in an easily accessible (i.e. scanning a code on the package), reader- and smartphone-friendly format (i.e. a web based format); most formats must support re-sizable text, multiple languages and searchable content.
- Lack of paper labelling from the commercial pack.
- Support for information in structured content (e.g. FHIR XML)
- Interoperability between systems (i.e. share of product information across wearables, ePrescription, and eHealth records).
Why is it important?
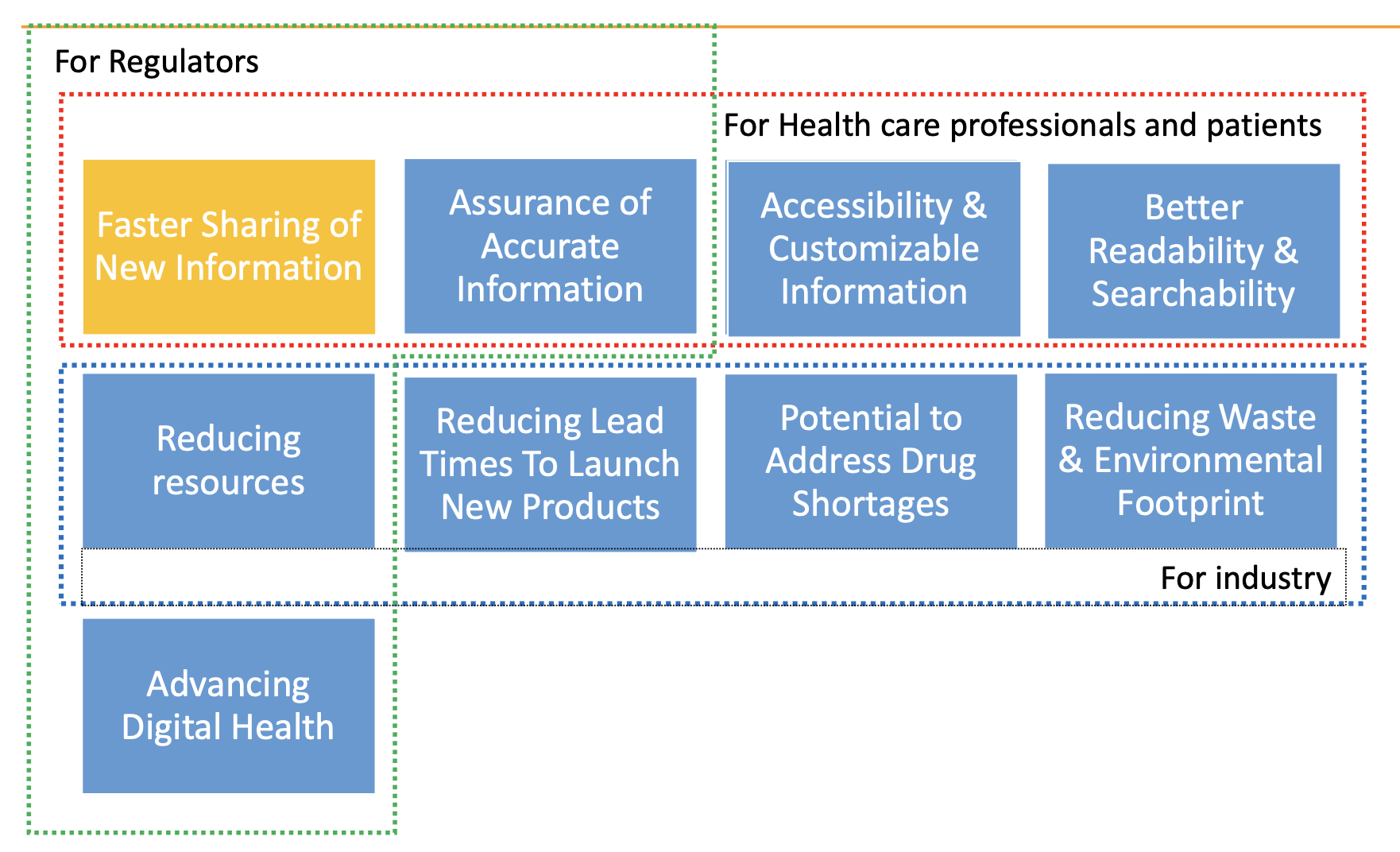
e-Labelling can improve:
- Accessibility and understanding of the most recent product information for patients and HCPs.
- Adherence.
- Patient outcomes.
- Process efficiency and reduce paper waste.
Benefits of e-Labelling
Concerns:
- Digital skills: individuals with insufficient digital skills, such as elderly people, may have difficulties in accessing product information in electronic format.
- Lack of internet access: in rural areas or in emergency situations internet access may be limited or not available.
The e-Labelling implementation in Japan
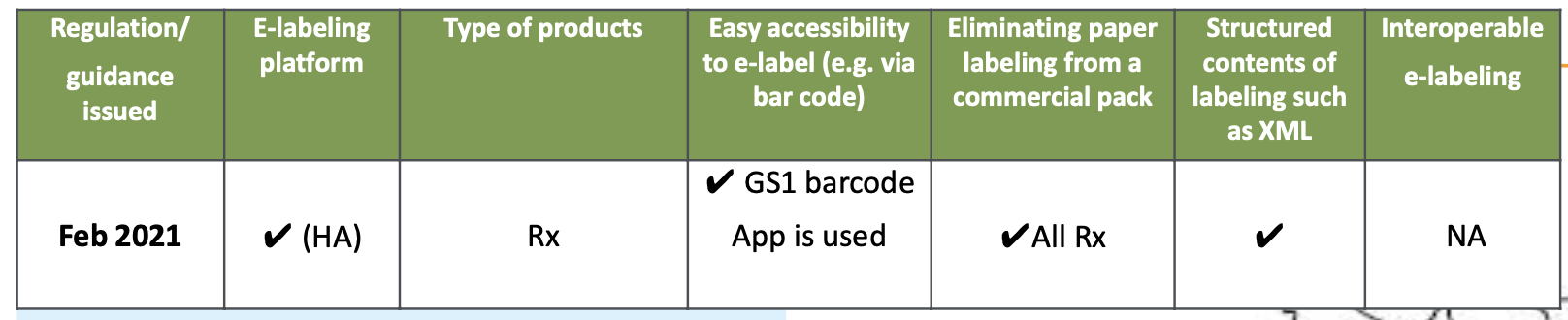
- PMDA has required SGML versions of the JPI (HCP labelling) for many years and has started to switch to XML in 2019.
- In December 2019 the "Pharmaceuticals and Medical Devices Act" was amended to introduce e-Labelling officially, replacing paper labelling and accompanying necessary scheme that enables all healthcare professionals access the up-to-date labelling information.
- The GS1 barcode needs to be printed on the outer box so that healthcare professionals can access labelling information. A mobile app for reading GS1 barcodes became available from May 2021.
- The enforcement of the "Pharmaceuticals and Medical Devices Act" amendment was implemented on Aug 1, 2021.
- After a 2-years transition period the requirement for paper labelling has been removed at the end of July 2023.
Print-on Demand and access to paper leaflets
The Marketing Authorisation Holder (in cooperation with a wholesaler, if needed) needs to provide a paper copy of the product leaflet upon request, specifically they are required to:
- Provide package inserts in paper format to medical institutions and pharmacies at the initial delivery of the products.
- Provide revised information in paper format to medical institutions and pharmacies in a timely manner.
Japan case: industry requirements for an e-Labelling implementation
They main tasks companies need to implement for an e-Labelling implementation are:
- Upload labels onto the PMDA website.
- Complete the link between the GS1 code and labelling information.
- Establish the process which includes updating the SOP to implement e-Labelling.
- Educate HCPs on how to use e-Labelling (app and web use).
Packaging Impact Assessment for e-Labelling
The roles of packaging:
Protect and preserve products during transportation, storage and distribution from initial containment to the end of the product consumption.
Critical roles of packaging insert:
- It is a tangible functional component of packaging.
- It aids steadiness of the product in container during transportation
- It can provide some degrees of light protection and thermal insulation
A Packaging Risk Assessment is a MUST Prior to any e-labelling implementation.
Japan: methods of browsing the latest package insert information
In 2022 the MHLW and the PMDA carried out a survey focused on, among others, the impact of the digitisation of package inserts, the main results from this survey are:
- 75.6% of hospitals and 48.1% of pharmacies visit the PMDA website to see the latest package insert information
- 47.9% and 55.0%, respectively of the respondent facilities use their in-house system such as the electronic medical record system and receipt computer system.
- 75.2% of hospitals and 71.4% of pharmacies also refer to paper package insert
- Only 1.7% of hospitals and 2.7% of pharmacies rely solely on paper package inserts
Outlook of e-Labelling Initiatives in the Asian markets
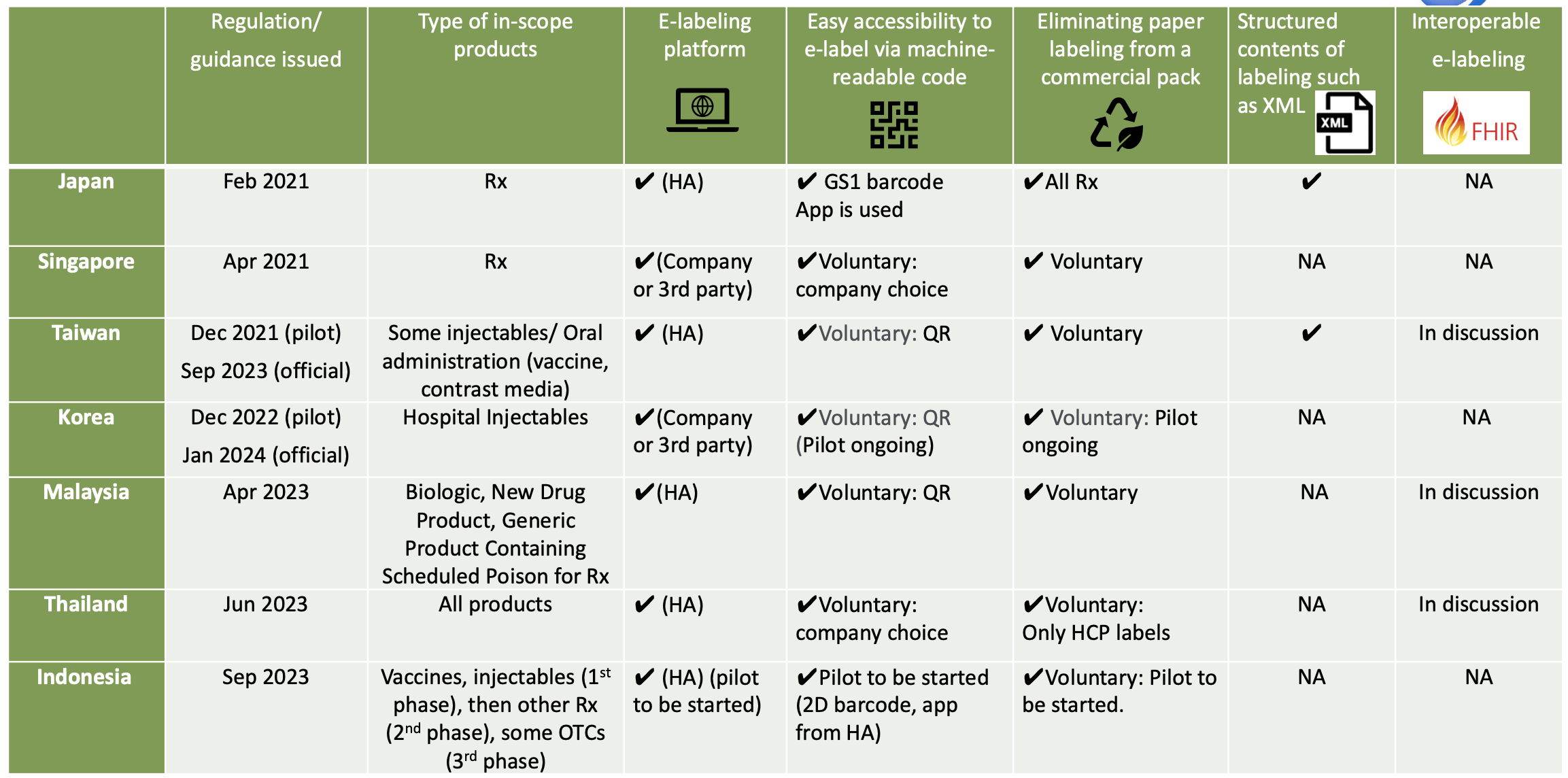
e-Labelling initiatives in Asia
In Thailand, the Thai FDA has officially announced e-labeling has been fully implemented for new registration via e- submission since June 23, 2023. e-labelling implementation is mandatory for new registration application submitted after the announced date. Accessibility code such as QR code should be indicated on the product carton. Once the accessibility code is scanned, the product information for HCPs and patients are available as the PDF files from the Thai FDA website. Paper labelling for HCPs can be removed due to the e-labelling implementation, but PIL is required for physical labelling.
In Singapore, HSA published HCP and patient labelling on their website, but their website is not used in connection with e-Labelling pilot study. The finalised guidance on e-labelling of Therapeutic Products* has been published and has taken effect from April 30, 2021. It is voluntary for companies to implement e-Labelling where physical labels need not to be distributed with the pack. Some companies have started e-Labelling pilot studies using QR codes and Data Matrix codes linking the pack to the PI.
In Malaysia, both HCP and patient labelling are published on Malaysia HA (NPRA) website. NPRA issued the Guideline on Electronic Labelling (e-Labelling) for Pharmaceutical Product in April 2023 and it was effective since May 1, 2023. E-labelling is defined as the provision of an approved product information that includes the package insert (PI) and/or Consumer Medication Information Leaflet (RiMUP) electronically via a machine readable Quick Response (QR) code on the outer carton/inner label of the product that links to the NPRA QUEST system.
In Indonesia, BPOM issued the Guidance on Implementation of e-Labelling pilot project on September 14, 2023. In the Pilot Project, e-labeling will be available on BPOM website via serialisation Barcode (QR or GS1 data matrix). BPOM mobile app will be used for e-Labelling purpose too. In Indonesia, GS1 data matrix has been implemented for serialisation purpose for some products, for which BPOM has already developed a mobile app and the function of e-Labelling will be added. Paper labels can be removed. The pilot project will last for 2 years.
Next steps
Having labelling information available as .doc (Word) or .pdf file is restrictive as these are “unstructured” formats. These files cannot be used “digitally” and do not allow for full exploitation of the possibilities offered by moving to a more web-friendly format.
Moving to a "structured format" like eLeaflet or HL7FHIR will offer a number of advantages:
- Opportunity for further digital transformation
- Link to Electronic Health Records
- Production of tailored (personalised) labels
- Automated creation of other materials
- Provision of real world evidence.