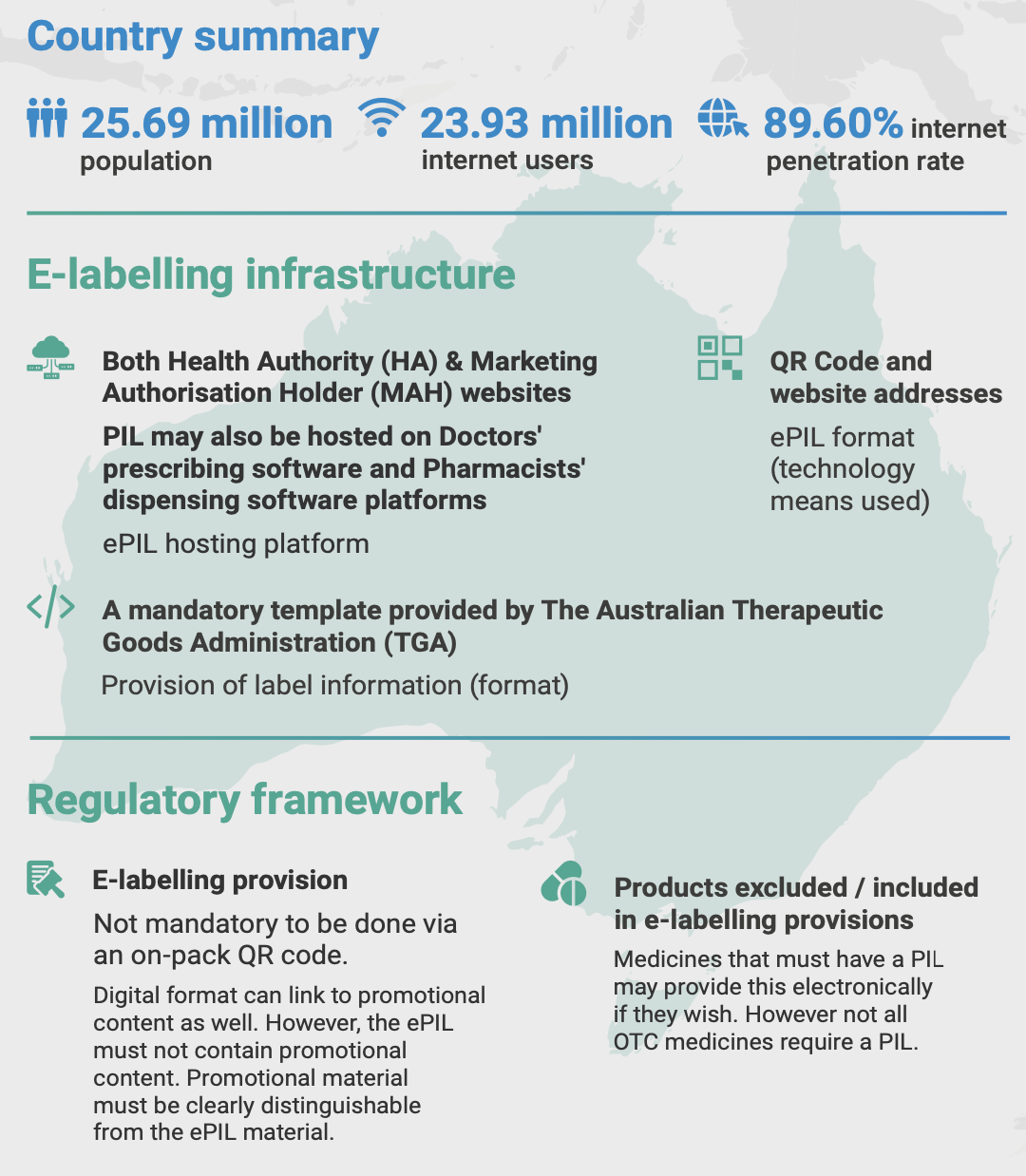
In Australia, providing accessible, clear, and accurate product information is essential for consumer safety and confidence. With the digitalization of healthcare, digital leaflets are becoming a viable alternative to traditional paper formats for prescription medicines, over-the-counter (OTC) products, medical devices, and supplements. The transition to e-labelling, regulated by the Therapeutic Goods Administration (TGA), has specific conditions under which digital-only formats can be used. Here’s an overview based on information from government and official sources.
Prescription Medicines
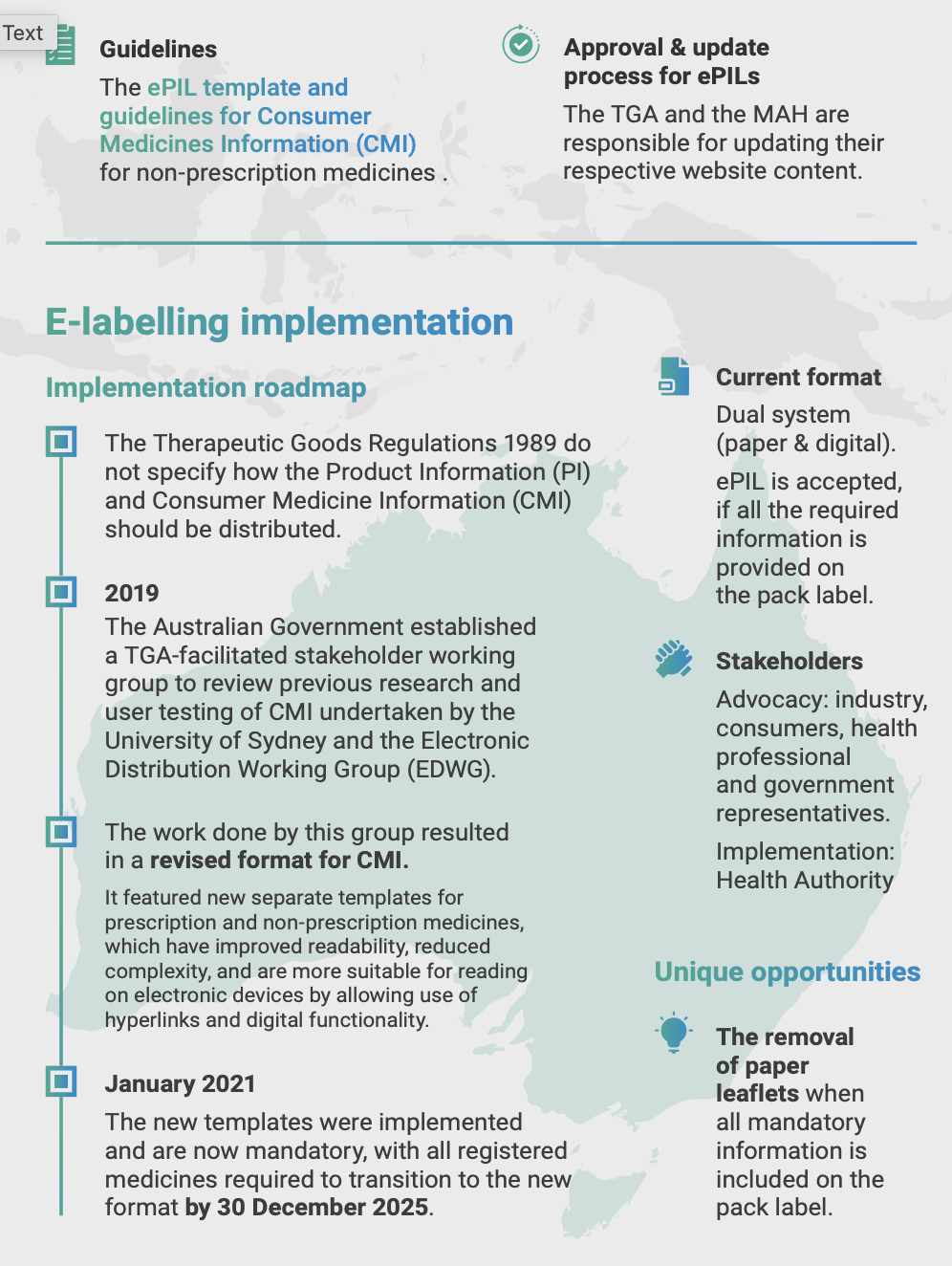
The TGA has introduced an Improved Consumer Medicine Information (CMI) Template to make information clearer and more accessible. For prescription medicines:
- Mandatory Digital Provision: The TGA allows digital CMIs to be made available via online databases and QR codes on packaging.
- Paper vs. Digital: While digital formats are encouraged, a paper version must still be provided if requested by the consumer. Digital-only provision is permissible if the product is exclusively distributed through digital channels (e.g., e-prescriptions).
- Timelines for Compliance: The new CMI format became mandatory for new entries from January 1, 2021, and all existing medicines must comply by December 31, 2025.
Source: TGA - Improved Consumer Medicine Information Template
Over-the-Counter (OTC) Products
For OTC products, the TGA allows digital CMIs under similar conditions:
- Accessibility Requirements: Consumers must have clear and immediate access to the digital leaflet. A QR code on the packaging linking to the document is a common approach.
- Dual Formats: Paper CMIs must still be available upon request.
- Adopting Digital-Only Formats: For OTC products distributed online or via app-based services, digital-only CMIs can be provided.
Medical Devices
The regulation of digital instructions for medical devices is also overseen by the TGA, with specific provisions outlined in the GSCF E-Labelling Implementation Guidelines:
- When Digital Leaflets Are Permitted: Digital instructions may be used as the primary information source if the device is distributed to professional users (e.g., hospitals) or directly to consumers with access to digital tools.
- Conditions: If provided digitally, users must be able to access the information without barriers (e.g., no paid subscriptions or difficult navigation).
- Paper Option: A paper leaflet must still be available upon request, ensuring inclusivity for all users.
Source: Electronic Instructions for Use - eIFU
Supplements
Supplements, regulated under complementary medicine rules by the TGA, can also adopt digital leaflets:
- Provision Standards: As with other product categories, supplements can include QR codes or links to digital leaflets, ensuring accessibility for consumers.
- Exemptions for Digital-Only: If supplements are sold exclusively through digital platforms, paper formats are not required. However, clear instructions on accessing the information must be provided.
Conditions for Digital-Only Leaflets
Across all categories, digital-only leaflets are allowed under the following conditions:
- Accessibility: The digital leaflet must be freely accessible, including offline options or downloadable formats.
- Consumer Awareness: Consumers must be informed about where and how to access the leaflet.
- Paper Option on Request: A paper version must be provided upon request unless the product is sold exclusively in digital channels.
- Regulatory Compliance: The digital leaflet must comply with TGA's requirements for content, format, and updates.
Future of Digital Information in Australia
The adoption of digital leaflets represents a step forward in ensuring sustainable and efficient healthcare communication. It also aligns with global e-labelling trends, reducing paper waste and providing real-time updates.
For manufacturers and sponsors, compliance with TGA guidelines and proactive consumer engagement are key to successfully implementing digital formats.
Explore more at: