Building Your Digital Leaflet Solution Using FHIR XML and the eLeaflet Platform
How To Deploy an ePI/FHIR XML solution
In this post we examine how to build a compliant electronic product information (ePI) / digital leaflet solution using FHIR XML, and how the eLeaflet™ solution from myHealthbox supports each of the three main implementation approaches.
Overview
Electronic product information (ePI) is now a key requirement for medicines and medical products. Standards such as HL7 FHIR define how both the structured XML part (i.e., machine-readable metadata, coded data) and the narrative content part (i.e., human-readable leaflet text) should be represented.
The goal is to have a system that:
- supports the structured data (codes, profiles, resources)
- supports the narrative content (XHTML/HTML for reading by humans)
- enables interoperability, regulatory compliance, multilingual support, and digital distribution
- ideally allows reuse of existing assets and integrates with internal systems.
Here we show how organisations can adopt one of three common approaches, and how eLeaflet™ can be used in each approach — for both structured and narrative parts.
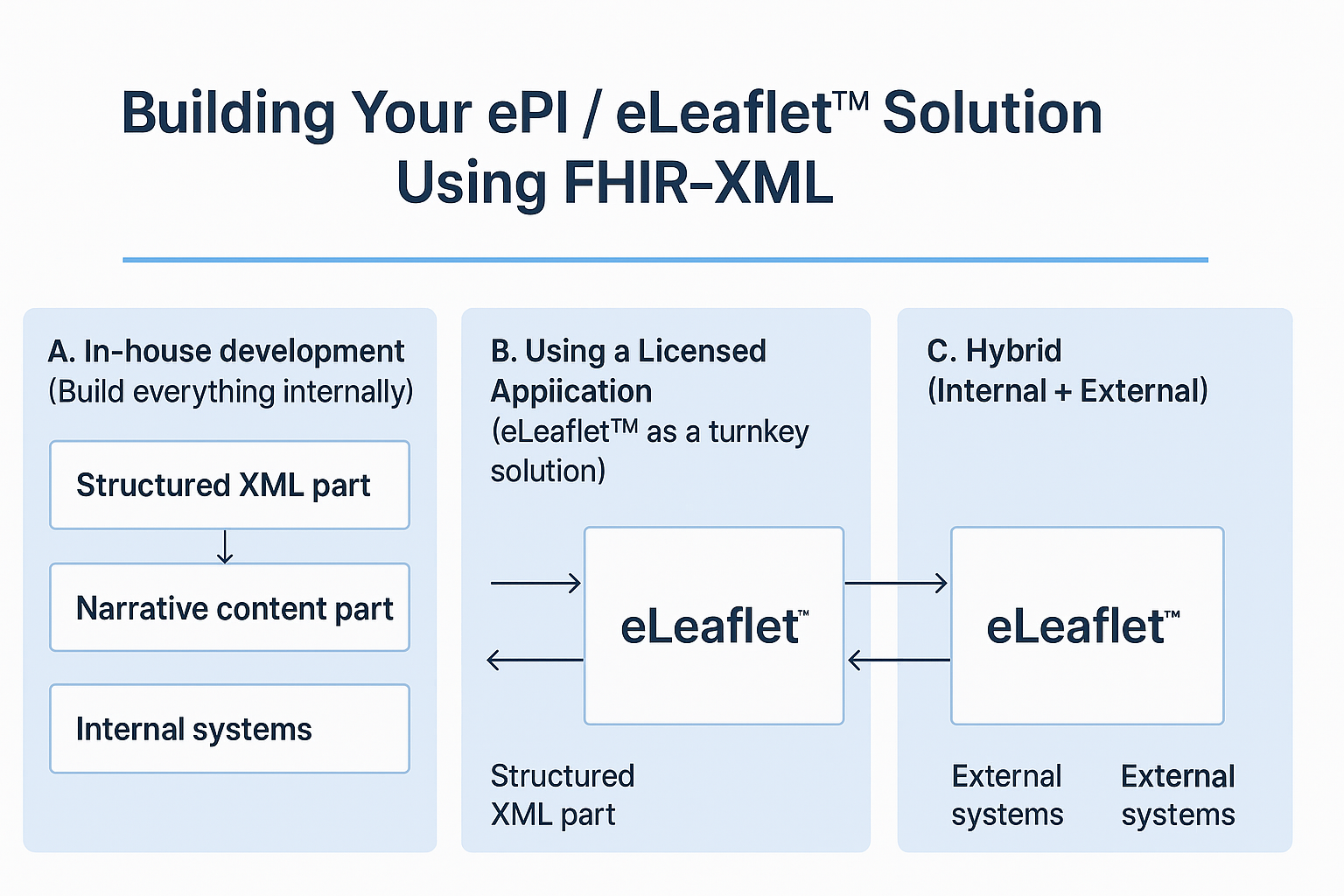
A. In-house development (Build everything internally)
In this approach, your organisation builds the ePI / leaflet solution entirely in-house. You design the FHIR profiles, build the XML pipelines, manage the narrative content workflows, and deploy the infrastructure yourself. You handle translation, versioning, regulatory updates and digital delivery.
When this approach is suitable
- You have strong internal IT, regulatory and content-management resources.
- You want full control over design, data flows, lifecycle, and integrations.
- You may already have legacy systems for leaflets, and you wish to modernise them.
How eLeaflet™ can help
Even when building in-house, you don’t need to start from scratch. The eLeaflet™ solution from myHealthbox supports:
- The structured XML part: eLeaflet™ provides a framework (profiles, mapping templates, validation tools) for generating FHIR XML output, so you don’t have to design everything from zero.
- The narrative content part: eLeaflet™ offers content-management workflows, multilingual support, version control, and delivery of the human-readable leaflet (web, mobile, PDF) that ties to the structured XML.
- Integration: eLeaflet™ is designed to plug into your existing content and regulatory workflows, reducing development effort.
So, adopting this “in-house build” approach with eLeaflet™ means you retain full control, but accelerate with a ready-to-use solution for both the machine-readable and human-readable parts.
B. Using a Licensed Application (eLeaflet™ as a turnkey solution)
This approach uses a licensed, vendor-provided software application (SaaS or on-premises) to implement the ePI / leaflet system. Your organisation configures it, perhaps customising some workflows, but the majority of technical development is done by the vendor.
When this approach is suitable
- You want to minimise internal development burden.
- You prefer a vendor-maintained, up-to-date system, including regulatory updates.
- You want fast deployment and lower upfront cost.
Where the eLeaflet™ solution fits
Here the eLeaflet™ product by myHealthbox is exactly the licensed application you need:
- For the structured XML part, eLeaflet™ delivers pre-built FHIR XML generation modules, profile compliance, validation, export to regulatory systems, and version tracking. Your team configures which resources, coding systems and templates to use, but the heavy lifting of XML generation and validation is handled.
- For the narrative content part, eLeaflet™ provides a workflow for content authors/translators to create, review and publish human-readable leaflets (in multiple languages), and automatically link or embed them with the structured XML package. It offers web-viewer, mobile viewer and archive functionality.
- The vendor (myHealthbox) takes care of updates (e.g., when regulatory formats change), security, hosting (if SaaS) and maintenance.
- Because eLeaflet™ is designed for this purpose, you avoid reinventing the wheel — you get a turnkey solution for both code/data and narrative content.
Therefore, the “licensed application” approach using eLeaflet™ is a fast, risk-reduced path to compliance, enabling you to focus on content, regulatory submissions and delivery, not building infrastructure.
C. Hybrid (Internal + External)
This hybrid approach combines internal resources plus vendor support. For example, you may keep content-creation internal but outsource XML generation and infrastructure, or you may manage the structured data in-house but use an external managed service for the narrative content viewer and delivery.
When this approach is suitable
- You want to retain some internal control (e.g., over translations or workflows) but outsource technical parts.
- You may already have some components (CMS, translation workflow) and want to plug in a specialist vendor for the rest.
- You wish to balance cost, control and speed.
How eLeaflet™ covers the hybrid use case
With eLeaflet™ you can flexibly adopt hybrid workflows:
- Use eLeaflet™ for the structured XML part, while keeping your internal content management team handling the narrative content. eLeaflet™ integrates with your existing CMS or translation system, exporting FHIR-compliant XML and linking to your narrative assets.
- Or conversely, you manage the narrative content internally (translations, human-readable versions, QA) but use eLeaflet™ as the vendor-managed service for packaging, validation, delivery and structured-data export.
- eLeaflet™ supports “plug-and-play” integration, so your internal team retains control over the parts you want, while leveraging vendor support for the rest.
Thus the hybrid approach with eLeaflet™ gives you the best of both worlds: internal control where it matters, vendor expertise where you need it.
Summary Table
| Approach | Focus | Role of eLeaflet™ solution by myHealthbox |
|---|---|---|
| In-house build | Full internal control, higher effort | eLeaflet™ serves as framework: structured generation + narrative management, reduces build time |
| Licensed application | Minimal internal dev, fast deployment | eLeaflet™ functions as turnkey: from structured XML to narrative content, maintenance included |
| Hybrid (Internal + External) | Balanced control & outsourcing | eLeaflet™ integrates: you choose which parts you manage, vendor handles the rest |
Why both “structured XML” and “narrative content” matter
It’s worth emphasising that modern leaflet/ePI solutions must support both dimensions:
- Structured XML part: This includes machine-readable data: coded resources (e.g., indication, dosage, active substance), versioning, metadata, regulatory identifiers. Using FHIR and XML ensures interoperability, data extraction, system integration, and future-proofing.
- Narrative content part: The human-readable leaflet (what patients and healthcare professionals see), multilingual text, readability, accessibility, regulatory formatting. This part must be linked with the structured data (for traceability, audit, version control) and delivered reliably via web/mobile/PDF.
The eLeaflet™ solution is designed to bridge both — not just generating XML, but also managing and delivering the narrative leaflet content — which is often overlooked in purely XML-centric approaches.
References
For more on how eLeaflet™ supports multilingual delivery, regulatory versioning and patient-facing interfaces, see our earlier blog posts on eLeaflet™ blog. For example:
- How eLeaflet™ delivers multilingual patient leaflets across the EU
- Regulatory version control in eLeaflet™: audit, traceability and change management
- Digital delivery of medicines information: web and mobile interfaces in eLeaflet™
Final thoughts
Whether your organisation is building entirely in-house, licensing a ready-made application, or deploying a hybrid model, it is critical to ensure you cover both the structured XML data layer and the narrative content layer.
The eLeaflet™ solution by myHealthbox provides a versatile and robust platform to accelerate your ePI / digital leaflet implementation — via framework, turnkey software, or hybrid integration — so you can focus on content, compliance and delivery rather than rebuilding infrastructure.
If you’d like to explore how eLeaflet™ could fit your specific workflow, content-management system or regulatory timeline, feel free to contact us and check out our detailed use-cases and customer stories on the eLeaflet™ blog.
Note: “eLeaflet™” is a registered trademark of myHealthbox.