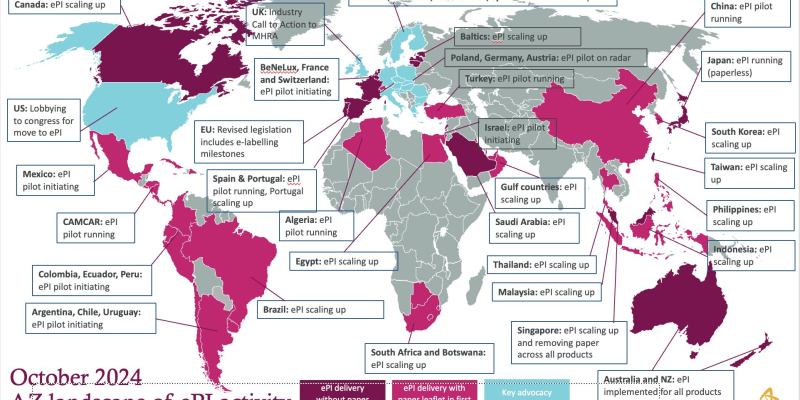
The debate is over: regulators worldwide agree that the future of patient and healthcare professional information is dig...
myHealthbox is proud to announce that its eLeaflet™ solution now supports Product Monographs in the Extensible Markup La...
In recent years, there has been frequent discussion about the possibility of replacing traditional paper instructions fo...
On June 27, the EU published Commission Implementing Regulation 2025/1234 ((Commission Implementing Regulation (EU) 2025...
In August 2024 the Jordan Food and Drug Administration (JFDA) announced a new mandate requiring the implementation of an...
On 8 January 2025 the Inter-Association Taskforce (IATF) on electronic Product Information (ePI), a collaboration betwee...
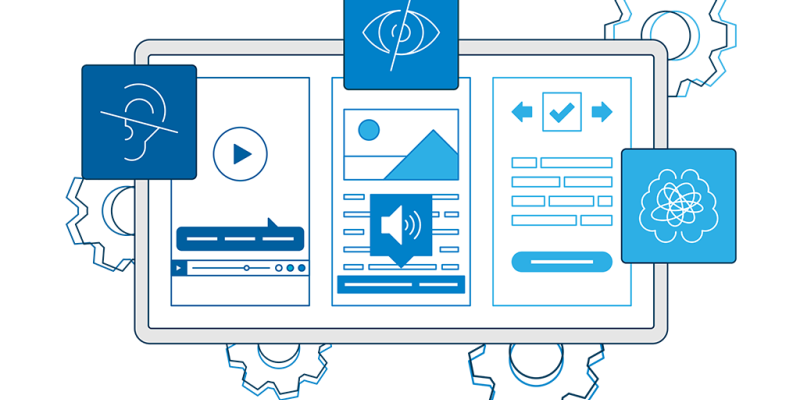
Digital accessibility is the practice of making digital content and services accessible to everyone, including people wi...
The Good Manufacturing Practice (GMP) is a system for ensuring that products are consistently produced and controlled ac...
Product information plays a pivotal role in ensuring patients’ understanding of their treatments while also supporting H...
The availability of the latest product information on publicly accessible websites can be an important first step in imp...
Indonesia is taking a significant step toward digital innovation in healthcare by introducing eLeaflets or electronic l...
Singapore’s Health Sciences Authority (HSA) is embracing innovation in healthcare communication through the use of elec...
In Australia, providing accessible, clear, and accurate product information is essential for consumer safety and confide...
As the world increasingly moves towards digitization, electronic Product Information (ePI) is becoming a vital aspect of...
The European Medicines Agency (EMA) has recently published a report detailing the outcomes of a one-year pilot project f...
Medical devices, perhaps more than any product, demand effective, efficient, and readily available user manuals. Instru...