Reducing Costs and Carbon: The Business Case for Digital Leaflets in South Korea
The Future of E-Labelling
South Korea is accelerating the digital transformation of healthcare, and medicinal product information is evolving with it. With recent regulatory updates and national pilot programs led by the Ministry of Food and Drug Safety (MFDS), digital leaflets are emerging as a practical alternative to traditional paper inserts.
Beyond regulatory modernization, digital leaflets offer pharmaceutical companies a compelling combination of cost savings, operational efficiency, and measurable environmental benefits.
A Regulatory Shift Toward Digital Product Information
South Korea formally enabled electronic product information through an amendment to the Pharmaceutical Affairs Act, enforced in January 2024. The update allows electronic information accessed via barcode or similar technology to replace paper leaflets for designated prescription medicines.
This regulatory change builds on earlier real-world use of electronic labeling, including during the COVID-19 period, and reflects the country’s broader strategy to modernize healthcare information delivery.
Pilot programs launched in 2023 and expanded in 2024 demonstrated the feasibility and benefits of digital leaflets across a growing number of pharmaceutical products.
Lower Costs Across the Pharmaceutical Lifecycle
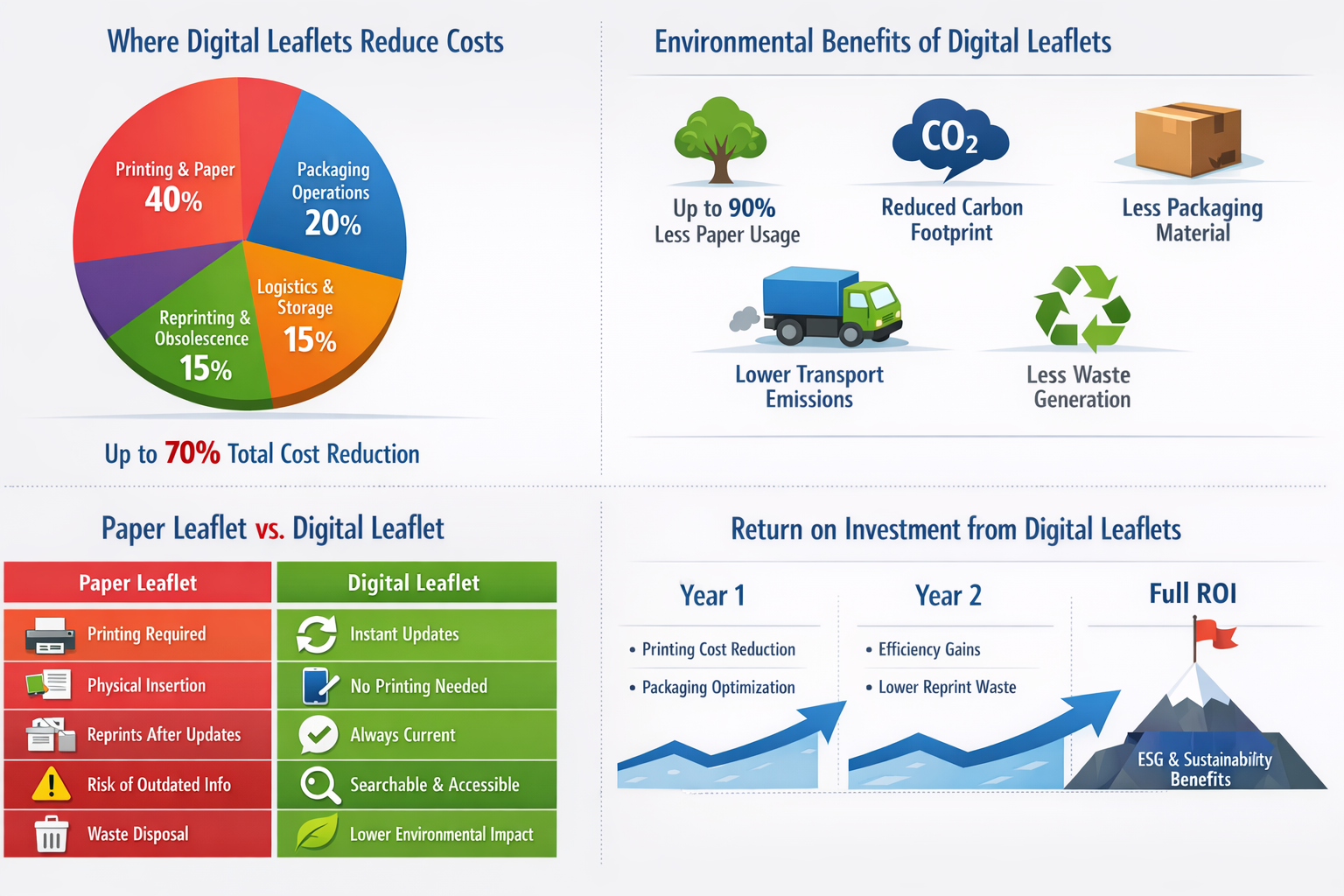
One of the most immediate and measurable benefits of digital leaflets is cost reduction throughout manufacturing, packaging, and distribution.
Traditional paper inserts generate continuous costs related to:
- Printing and paper procurement
- Packaging and leaflet insertion
- Storage and logistics
- Waste management and disposal
- Reprinting following regulatory updates
Digital leaflets eliminate or significantly reduce these recurring expenses.
Industry benchmarks indicate that switching to digital leaflets can reduce leaflet-related costs by 30–70%, depending on product volume and update frequency. Products requiring frequent labeling updates benefit the most, as digital information removes the need to discard outdated printed stock.
Packaging Optimization and Manufacturing Efficiency
South Korea’s pilot projects demonstrated tangible manufacturing improvements. Removing paper inserts allowed smaller carton sizes and optimized packaging, reducing excess material and improving packing efficiency.
In large-scale pharmaceutical production, even small packaging reductions translate into:
- Lower material procurement costs
- Increased pallet density and shipping efficiency
- Reduced transport weight and fuel consumption
- Improved production throughput
These operational efficiencies contribute directly to measurable ROI.
A Greener and More Sustainable Solution
Sustainability is becoming a core priority across the pharmaceutical industry, and digital leaflets offer a practical way to reduce environmental impact.
Replacing paper inserts helps:
- Reduce paper consumption dramatically
- Lower energy use in printing and manufacturing
- Decrease packaging material usage
- Reduce transport-related emissions
- Minimize waste generation
South Korea’s e-labeling pilot specifically identified reduced carbon footprint as a key advantage of digital labeling.
For companies with ESG and sustainability commitments, digital leaflets represent a scalable and measurable environmental improvement.
Better Information, Better Accessibility
Digital leaflets not only reduce costs and environmental impact — they also significantly improve access to information for healthcare professionals and patients.
Electronic labeling enables:
- Always up-to-date product information
- Improved readability compared to folded paper leaflets
- Searchable content for faster information retrieval
- Digital accessibility features
Future regulatory developments in South Korea include integration with audio and sign-language formats, further improving inclusivity and accessibility.
Operational and Compliance Advantages
Digital leaflets strengthen regulatory compliance and operational performance by enabling:
- Instant updates following regulatory changes
- Reduced risk of outdated information in circulation
- Improved traceability and version control
- Simplified multi-market deployment
- Lower recall and reprint risks
These benefits reduce hidden operational costs while improving quality and compliance outcomes.
Expanding Adoption Across Industries
Digital labeling in South Korea is expanding beyond prescription medicines. Electronic information initiatives are already underway for:
- Risk Management Plan (RMP) materials
- Cosmetics
- Food products
This broader adoption signals a clear national trajectory toward digital information ecosystems across regulated industries.
The Business Case for Digital Leaflets
For pharmaceutical companies operating in South Korea, digital leaflets deliver measurable business value:
Financial Impact
- 30–70% reduction in leaflet-related costs
- Lower packaging and logistics expenses
- Reduced waste and reprint costs
Operational Impact
- Faster labeling updates
- Improved manufacturing efficiency
- Simplified global compliance
Environmental Impact
- Significant reduction in paper use
- Lower carbon emissions
- Support for ESG and sustainability reporting
Digital leaflets are no longer just a regulatory alternative to paper — they are a strategic lever for cost optimization, sustainability, and digital transformation.
The Future of e-Labeling in South Korea
While challenges remain — such as platform standardization, barcode integration, and expanding product coverage — the direction is clear. Digital leaflets are becoming a key component of South Korea’s healthcare digitalization strategy.
As adoption grows, pharmaceutical companies that embrace digital leaflets early can benefit from:
- Long-term cost reductions
- Stronger sustainability positioning
- Improved digital readiness
- Competitive advantage in regulated markets
Digital leaflets are shaping the future of pharmaceutical information in South Korea — making it smarter, greener, and more efficient.
Technical and Regulatory Deep Dive: e-Labeling Framework in South Korea
As digital leaflets gain traction in South Korea, understanding the technical and regulatory framework behind e-labeling is essential for pharmaceutical companies planning implementation or market expansion.
Legal Foundation for Digital Leaflets
The legal basis for electronic labeling in South Korea comes from the amendment to the Pharmaceutical Affairs Act (PAA), enforced on 2 January 2024. The amendment permits the electronic provision of product information via barcode or equivalent digital access methods as a substitute for paper package inserts for designated prescription medicines.
However, implementation is not automatic. Products must be specifically designated by the Ministry of Food and Drug Safety (MFDS), and companies must comply with defined technical and operational requirements.
Scope of the e-Labeling Pilot Programs
South Korea introduced e-labeling through structured pilot programs to evaluate safety, usability, and technical feasibility before full-scale adoption.
2023 Pilot
- 10 Marketing Authorization Holders (MAHs)
- 27 pharmaceutical products
- Focus on hospital-administered injectable medicines
- Option to keep or remove paper leaflets
- Contingency planning required for digital access interruptions
2024 Expanded Pilot
- 109 products from 27 MAHs
- Continued focus on hospital-administered injectables
- Inclusion of biopharmaceuticals
- Exclusion of emergency-use and home-administered products
- Digital formats accepted: XML and PDF
- Platforms may be MAH-operated or vendor-provided
These pilots aim to validate real-world implementation, user accessibility, and regulatory compliance.
Technical Implementation Requirements
Pharmaceutical companies implementing e-labeling in South Korea must address several technical elements:
1. Digital Access Mechanism
- Product information must be accessible via barcode, QR code, or equivalent
- Codes must link directly to current approved product information
- Reliable uptime and accessibility must be ensured
2. Content Format and Structure
- Accepted formats include structured XML and PDF
- Content must match the approved labeling version
- Version control and traceability are required
3. Platform Infrastructure
- Companies may use their own platform or approved vendor platforms
- Systems must support secure hosting and regulatory traceability
- Integration with MFDS systems is expected to expand over time
4. Business Continuity
- Contingency plans must ensure access to information during connectivity failures
- Backup access methods may be required in healthcare settings
Accessibility and Patient-Centric Requirements
South Korea is progressively strengthening accessibility requirements for digital health information.
A separate amendment to the Pharmaceutical Affairs Act (effective July 2024) mandates:
- Braille labeling for certain medicines
- Digital codes linking to audio and sign-language versions of product information
These requirements reinforce the importance of inclusive digital content and accessible information delivery.
Integration with MFDS Digital Ecosystem
Future implementation of e-labeling is expected to integrate with broader MFDS digital systems, including:
- Drug Utilization Review (DUR)
- Risk Management Plan (RMP) materials
- Labeling revision history and traceability
- Production and import tracking
- Health insurance and post-market surveillance systems
This integration will strengthen regulatory oversight, data consistency, and lifecycle management of pharmaceutical information.
Operational and Regulatory Challenges
Despite strong progress, several challenges remain:
- Managing multiple barcodes for labeling, accessibility, and distribution
- Standardization of platforms across MAHs and regulators
- Expansion beyond hospital-administered products
- Ensuring consistent user awareness and adoption
- Balancing digital access with patient safety requirements
Regulators continue to evaluate these factors as part of the phased rollout of e-labeling.
Strategic Considerations for Pharmaceutical Companies
Companies planning digital leaflet implementation in South Korea should prepare for:
- Regulatory designation requirements
- Technical platform readiness
- Content version control and compliance
- Accessibility and multilingual content
- Integration with broader regulatory digital systems
Early preparation enables smoother regulatory alignment and faster adoption as e-labeling expands beyond pilot programs.
Enabling Digital Leaflets with the eLeaflet™ Platform
Implementing digital leaflets at scale requires a secure, compliant, and regulatory-ready infrastructure. The eLeaflet platform provides pharmaceutical and healthcare companies with a complete solution to create, manage, and distribute official digital product information globally.
Learn more: https://eleaflet.eu
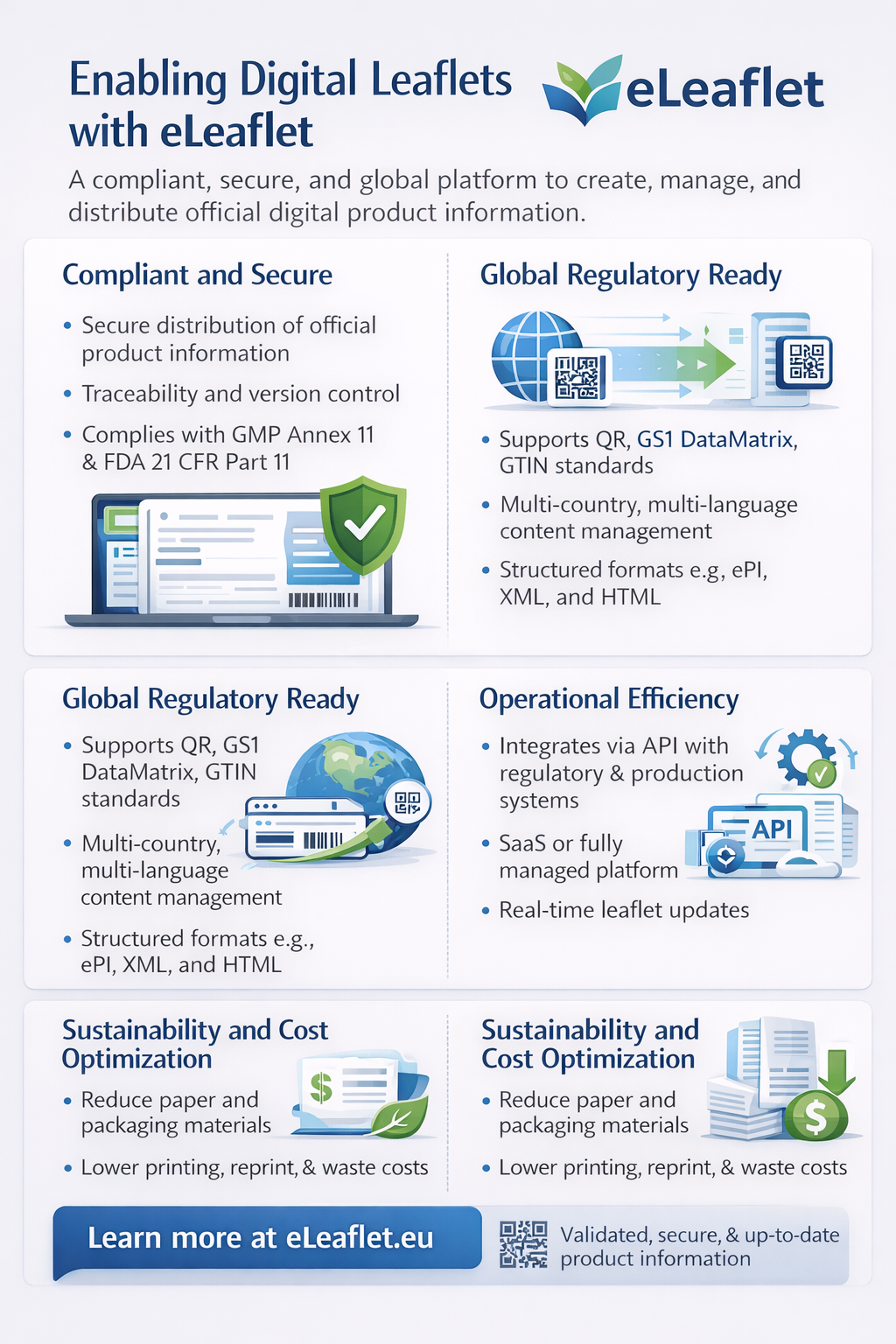
A Compliant and Secure Digital Leaflet Infrastructure
The eLeaflet solution is designed specifically for regulated healthcare environments, enabling companies to distribute verified, approved, and up-to-date product information in digital format while meeting pharmaceutical-grade quality and security standards.
Key capabilities include:
- Secure distribution of official product information
- Digital signature and content integrity protection
- Full traceability and version control
- Compliance with GMP Annex 11 and FDA 21 CFR Part 11 requirements
- Secure hosting and controlled document lifecycle
This ensures that healthcare professionals, regulators, and patients always access the correct and approved version of product information.
Built for Global Regulatory Environments
The platform supports digital leaflet deployment across multiple markets and regulatory frameworks, enabling companies to manage product information centrally while meeting local requirements.
Core regulatory-ready features:
- Support for structured formats such as ePI, XML, and HTML
- Compatibility with global barcode standards (QR, GS1 DataMatrix, GTIN, Digital Link)
- Multi-country and multi-language support (45+ languages, 90+ localizations)
- Stable and secure digital access via QR or barcode scanning
- Platform validation aligned with pharmaceutical compliance standards
This allows organizations to scale digital labeling strategies globally while maintaining regulatory control.
Operational Efficiency and Seamless Integration
The eLeaflet platform simplifies the transition from paper to digital by minimizing technical complexity and enabling rapid deployment.
Operational advantages include:
- SaaS or fully managed deployment options (EaaS)
- API integration with regulatory, packaging, and production systems
- Real-time leaflet updates without reprinting or repackaging
- Multi-lot and multi-language content management
- Centralized content governance and lifecycle management
These capabilities help reduce operational burden while improving speed, accuracy, and scalability.
Supporting Sustainability and Cost Optimization
Digital leaflets delivered through the eLeaflet platform contribute directly to sustainability goals by reducing paper consumption, packaging materials, and logistics-related emissions. At the same time, companies benefit from lower printing, reprint, and waste management costs, supporting both ESG objectives and operational efficiency.
The transition from paper to digital product information is accelerating worldwide. Platforms such as the eLeaflet™ solution by myHealthbox enable pharmaceutical companies to implement compliant, scalable, and future-ready digital leaflet strategies while improving efficiency, sustainability, and patient access to information.