An e-PIL pilot project in Belgium & Luxembourg
More Eu countries experimenting with eLeaflet solutions
The e-PIL pilot project launched in Belgium & Luxembourg which is now at its second call for candidates, open from 17 August 2020 up to 15 September 2020, proposes a switch from paper PIL to e-PIL in the packaging of selected approved medicinal products available on the Belgium and/or Luxembourg market but restricted to hospital use (there may be no direct delivery of the concerned medicinal products to the patient by the hospital pharmacy which means the products can only be used within the hospital and administered by the hospital personnel).
In summary for the products which are part of the pilot the manufacturers can remove the paper leaflet from the packaging and only provide it electronically via one or more trusted sources.
The project initially set to last for a limited period of 24 months (project initially started on 01 August 2018), has now been extended to 48 months (until 01 August 2022).
Objective of the pilot
This pilot aims at demonstrating that the electronic PIL is equivalent to the paper PIL to provide the information necessary for a safe and effective use of medicines to the patients and the healthcare professionals in an hospital setting.

Communication
While the paper leaflet has been removed from the product packaging plenty of information about the project and the availability of the information in electronic format has been provided to the hospitals via their responsible hospital pharmacist.
The name of a dedicated contact person for each marketing authorization holder was also provided in case of questions about those products (please note that for most products a dedicated medical information contact is always provided to hospitals and their responsible pharmacist so in reality no additional resources where needed to cover this requirement).
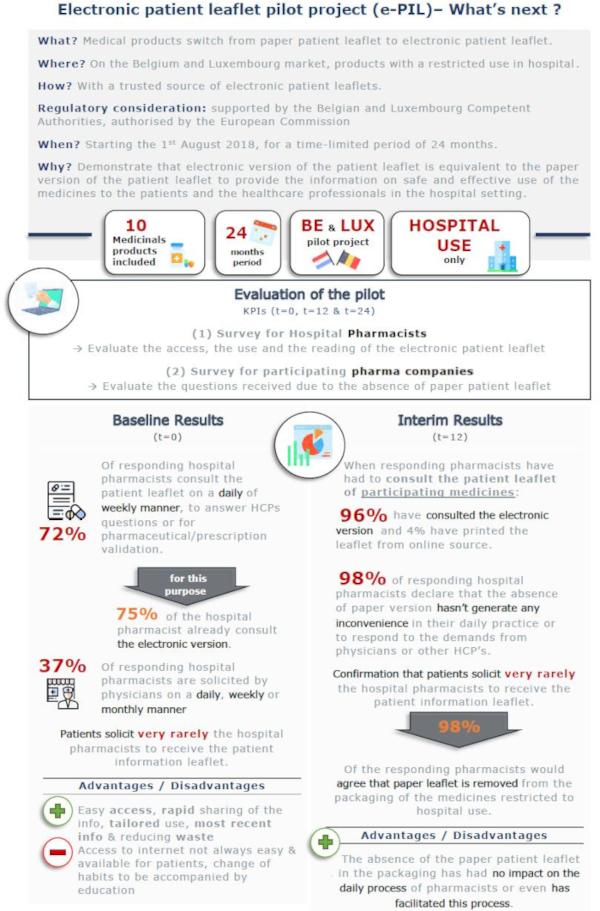
Interim results
Thanks to the positive results observed in the intermediate analysis of the pilot project, the Belgian and Luxembourg’s authorities agreed to request an extension of the project to the European authorities, which has been approved for an additional period of 24 months (until 01 August 2022) and for inclusion of more products.
Preliminary results after the first phase of the project are very encouraging:
- the consultation of the patient leaflet in a digital format went from 75% before the study (clearly indicating that the digital format is already the preferred format for HCPs) to 96% after the study.
- only 4% of respondents printed the digital leaflet from the online source
- 98% declared that having only a digital version of the leaflet did not generate any inconvenience in their daily practice
- 98% of consulted pharmacists agreed that the paper leaflet could be removed from the packaging of medicines restricted to hospital use
More information about the eLeaflet solution from myHealthbox is available on the eLeaflet website